Back in the day (2013), the idea that a universal item number (UDI) would be established for all medical products, creating a "universal language" across healthcare, generated quite a buzz in the industry. The FDA GUDID, like its cousin, the FDA NDC (National Drug Code) directory, would revolutionize healthcare supply chain data management. Unfortunately, the journey has been riddled with a litany of challenges preventing its growth and adoption. Lack of participation by the manufacturers, and data quality issues have created a delay in the adoption of these important standards.
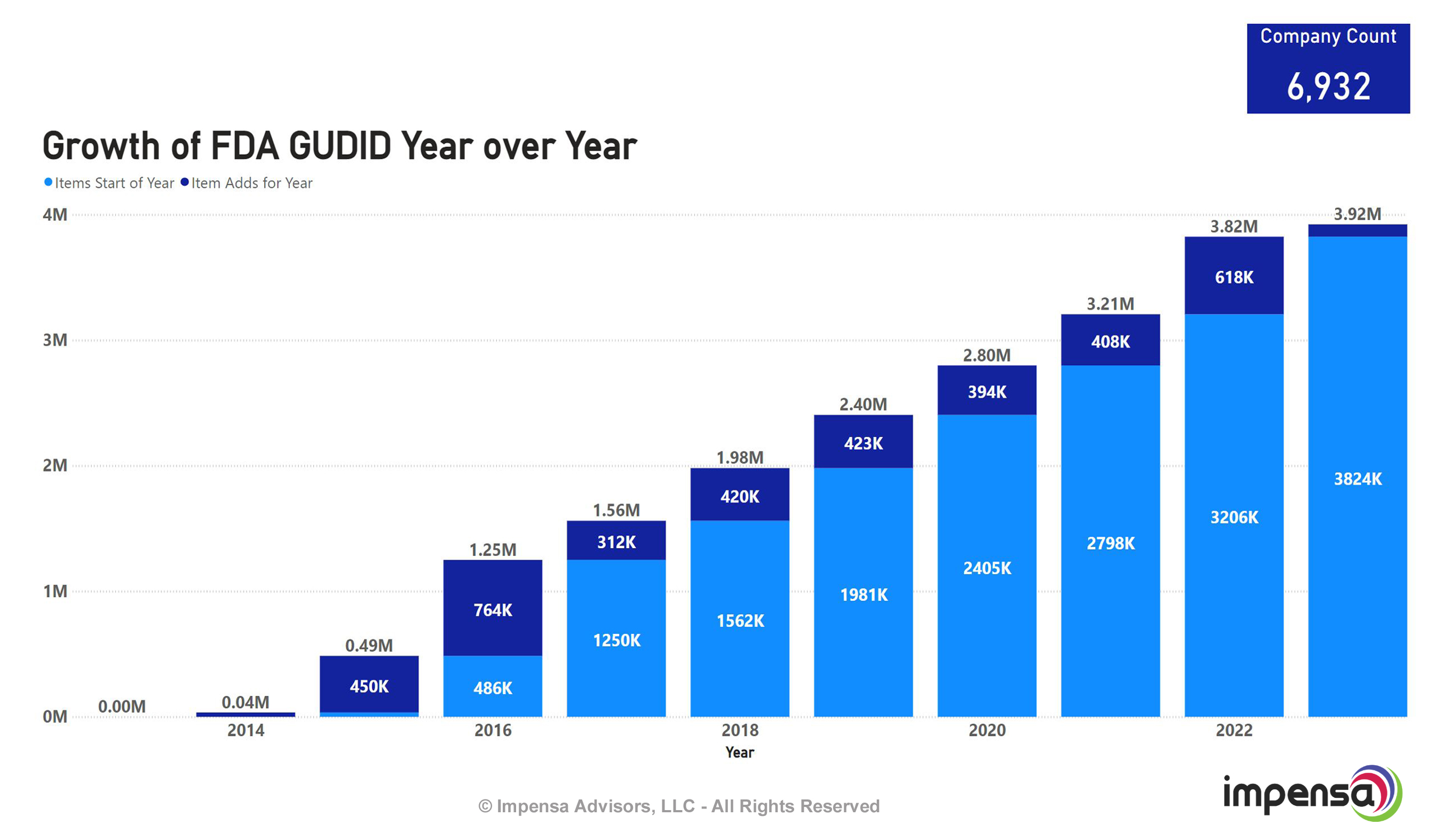
Reviewing the headline graphic, you can see that over the past 10 years since its inception, the numbers have grown steadily to almost 4 million items and 7,000 companies. For those of us observing, it feels like interest started to fall as this database hit 2 million items because too many suppliers and items were missing, and supplier deadlines were pushed out. Fast forward to 2023 and this database has really grown to a point where we feel it's hit the critical mass needed for providers to re-evaluate and consider ways to utilize this valuable data to lower their data management costs and enable their own business intelligence.
Background
The Global Unique Device Identification Database (GUDID) is a database established back in 2013 that is maintained by the US Food and Drug Administration (FDA) and contains information on medical devices distributed in the United States. GUDID is a central repository for device identification and product data that can be accessed by healthcare providers, patients, insurance companies, and other stakeholders.
The GUDID Database was implemented as part of the FDA mandate requiring a Unique Device Identifier (UDI) registration and transparency of all medical devices sold in the United States. So, basically a universal ID is created at each packaging level for each medical device or product.
The National Library of Medicine (NLM), in collaboration with the FDA, has created the AccessGUDID portal to make device identification information in the GUDID available for everyone, including patients, caregivers, health care providers, hospitals, and industry. This portal is updated every business day: https://accessgudid.nlm.nih.gov/
Challenges
Although there are many benefits that GUDID offers (which we will discuss in detail in the next blog of this FDA GUDID series), it has not been widely accepted by hospitals and health systems. Below, we present several of the perceived challenges that have caused the lack of adoption of this "universal" item code language.
Limited participation by some manufacturers
One major challenge facing GUDID adoption has been the limited adoption of the system by medical device manufacturers. While the FDA requires manufacturers to submit data to the GUDID for certain devices, many manufacturers have been slow to comply, leading to gaps in the database and making it difficult for hospitals to rely on the data provided by GUDID. This is especially true for many commodity manufacturers of medical products. However, this number has increased drastically in the past few years to almost 7,000 companies participating.
Data quality issues
Another challenge with GUDID adoption has been the issue of data quality. While GUDID is intended to provide standardized data on medical devices, the quality of the data can be highly variable, with errors, inconsistencies, duplicates, and missing information. This has made it difficult for hospitals to rely on the data provided by GUDID and has limited its usefulness for value analysis and data maintenance. In recent years however, the GUDID team has worked to educate and improve the supplier uploads, so the issues are drastically improving.
Integration challenges
GUDID is a separate system from hospital information systems and supply chain management platforms, which makes it difficult to integrate the GUDID data into existing workflows and processes. This can create additional work for hospital staff and limit the usefulness of GUDID data for value analysis and other applications. Ironically, it's difficult to connect the industry standard data to hospital data because of differences in manufacturer or vendor names and variability of catalog numbers representing the same item. This is probably the biggest barrier to adoption, however there are solutions available to remove this barrier.
Lack of awareness and training
Due to the challenges experienced over the long journey which caused a heavy decline in the interest of adoption, there may be a lack of awareness of the current state of the FDA GUDID and the benefits it provides. Hospital and health system leaders and decision-makers need to be educated on the current state and the benefits it can provide their organizations. Without a clear understanding of the benefits and how it can be used to improve interoperability, value analysis, and other processes, hospitals will continue to be reluctant to adopt or make it a priority.
Conclusion
By leveraging the power of GUDID, hospitals can make more informed purchasing decisions, reduce costs, and improve patient outcomes, ultimately delivering greater value to their patients and their bottom line.
Stay-tuned for the next blog of the series:
The Evolution of the FDA GUDID:
Part 2 - The Benefits and Uses of the FDA GUDID.