The Global Unique Device Identification Database (GUDID) is a database maintained by the US Food and Drug Administration (FDA) that contains information on medical devices distributed in the United States. GUDID is a central repository for device identification and product data that can be accessed by healthcare providers, patients, and other stakeholders.
Overview
While there has been a substantial increase in participation by the medical suppliers over the past several years, the adoption and utilization of this data by provider organizations has been lagging. Aside from the lack of awareness of how the GUDID has grown, providers have been slow to adopt due to an uncertainty around the process itself, and the effort involved.
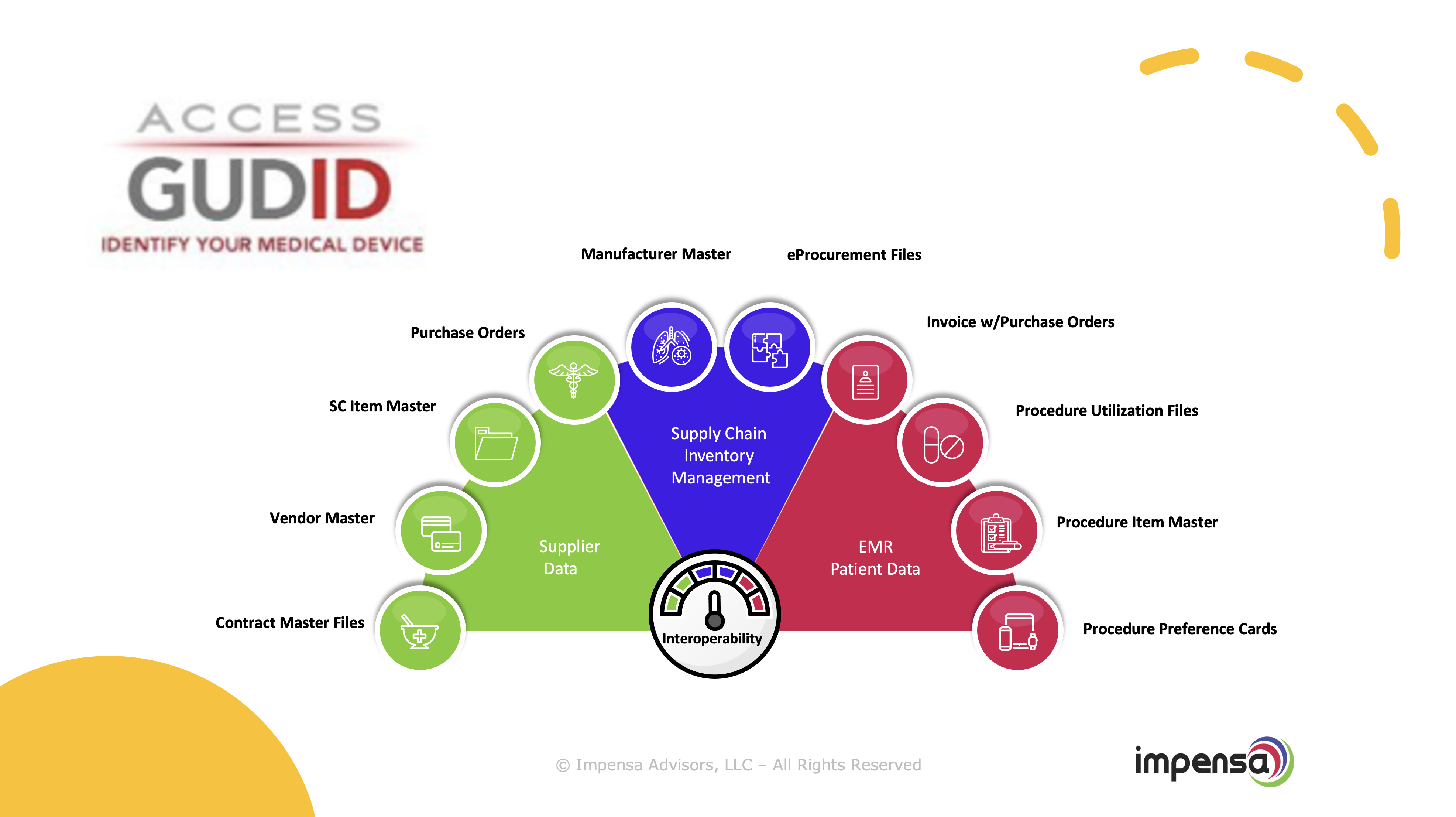
Needless to say, providers experience a litany of challenges with their medical product data. The lack of utilizing a “universal language” for product codes creates a lack of interoperability, a lack of transparency, data silos and dirty data. The pain and challenges are well established in this industry.
Leveraging the GUIDID data offers an immense opportunity for the provider to address these challenges by providing the standardization necessary in their master data to support the interoperability across their Supply Chain, ERP, EMR, and Revenue Cycle.
Benefits of Adopting the FDA GUDID information
True device identification
One of the primary benefits of GUDID is the improved transparency and accuracy of device identification and product data through a unique Primary UDI number.
Regardless of how many variations of a "hand-typed" (dashes/no dashes, leading zeroes/no leading zeroes, different manufacturer names) items you have duplicated in your different system or even different hospitals, they can all be unified and managed across all systems and in analytics down to a single Primary UDI number. This provides better visibility to manage analytics, sourcing, contract management, price parity, integration, and other challenges hidden by data without standard identification.
Supply Chain Management and Inventory Control
GUDID provides a standardized system for identifying and tracking medical devices which can help healthcare providers manage their inventory more effectively, enabling barcoding, improving patient safety, and extending purchase order automation.
Clinical Integration - Procedure Product, Cost, Revenue Capture
GUDID standardization enables the tracking of medical devices at the Point of Use. This standardized data can be used to quickly identify items outside of the Item Master or EMR product master such as "Trunk Stock” and provide content to barcode scanning systems to ensure proper capture. This can also eliminate research and manual typing by clinical and operations staff.
Simplified Compliance
The GUDID can help manufacturers simplify compliance with FDA regulations by providing a standardized method for submitting device identification data used in procedures.
Centralized Source and Master Data Management
GUDID provides a centralized “source of truth” for information about medical devices. This can be accessed by a wide range of stakeholders within the hospital that use product information. It’s unrealistic and almost impossible for a provider team to know which items they may need to use in a procedure and be able to maintain key information on millions of items in their item master, so the GUDID database provides the ability to unify all provider data, as well as supplement items outside of their master data set.
Improved Business Intelligence
GUDID standardized data can enable extensive transparency and enrichment of product data. This enhances analytics to manage hospital spend and to provide clarity and visibility into products utilized on patient procedures. Most providers suffer blind spots in their analytics when 20-30% of their patient procedure spend is hidden in invoice only/trunk stock spend.
Post-Market Surveillance
The GUDID database includes information on device performance, adverse events, and recalls, which can be used by regulatory agencies and manufacturers to identify potential safety issues and take corrective action as needed. The more that healthcare providers can capture and share the GUDID information, the more data analysis will be available to study.
Conclusion
In summary, the FDA GUDID offers a valuable resource with many benefits for healthcare providers, enabling them to address data challenges, streamline processes, improve patient safety, enhance compliance, and gain valuable insights through standardization and centralized device identification and product data. The adoption of the GUDID by hospital providers can lead to significant improvements in the healthcare supply chain industry.
Stay-tuned for the next blog of the series:
The Evolution of the FDA GUDID:
Part 3 - Utilizing the FDA GUDID to Support Interoperability and Analytics